Nicklaus Children's Research Institute (NCRI) is celebrating the close of a remarkable year of growth and is excited to share the vision for the upcoming year. Committed to promoting quality research at Nicklaus, the team at NCRI is driven by the positive impact it has on pediatric medical care and treatment.
Commenting on the impact of clinical research, Jennifer Lilley, Executive Director of NCRI, said “When our patients participate in an interventional clinical trial, they are moving science forward and what is considered experimental today may become tomorrow's standard of care.”
Expanding on the impact that research has and highlighting benefits of research for Nicklaus patients, Dennis Golbourne added that “The standard of care for many diseases is well known but clinical trials provide a treatment option that is outside of standard of care.” For patients whose condition is rare, has no effective standard treatment, or is less responsive to standard of care treatments, research makes new treatment options available. Jennifer looks forward to watching the “NCRI continue to grow and provide cutting-edge therapies to our patients through clinical trial research.”

Thanks to a new NCRI leadership team curated by Dr. David Seo, Senior Vice President, and Jennifer, 2024 was marked by notable changes to internal processes. A strategic transformation of the NCRI took place as leadership focused on improving quality and compliance through more efficient and effective internal processes. Jennifer is excited to head this group of exceptional leaders as they “continue to engage in a transformational journey to exceed standards and achieve industry-leading results through robust clinical research.”
Throughout their first year as a leadership team, Rebecca Jones, Director of Research Operations, Xonia Cerro, Director of the Office of Sponsored Programs, and Dennis Golbourne, Director of Research Administration, worked collaboratively to implement new initiatives. Jennifer said that “Together they have centralized research services and operations which has streamlined workflows for processing contracts and billing services quickly and efficiently, more research support in our clinics, and faster days to activation for clinical trials.”
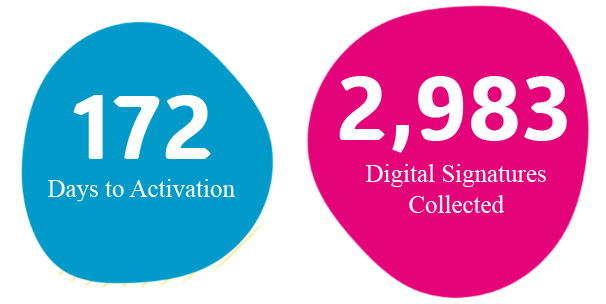
Leveraging technology was arguably the most impactful NCRI initiative in 2024. Spearheaded by Dennis Golbourne, implementation of a clinical trials management system (CTMS), and Florence, an e-regulatory binder application, promoted efficient internal processes, improved file accessibility, and made it possible to accurately track research quality, compliance, and progress. Prior to implementation of Florence, collection of wet-ink signatures was a time consuming activity, taking hours of valuable staff time. This process was reduced to a matter of minutes by collecting signatures digitally through Florence. 2,983 digital signatures collected in 2024 represents countless hours of productivity returned to research staff.
Previously, subject and study progress tracking was distributed across multiple NCRI staff members. Variations in individual record keeping practices lead to delays and inaccuracies in progress reports resulting in longer response times to deviations and complicated the billing process. In 2024, implementation of the CTMS enabled real-time reliable tracking of study status, enrollment, and subject visits. This more efficient system standardized tracking practices across NCRI and supported timely reporting, responses to deviations, and billing.
Orchestrated by Rebecca Jones, staff in the operations team have implemented several initiatives aimed at streamlining procedures in 2024. One of the most recognizable outcomes of these efforts is the reduction in the time required to open a new study. By centralizing this process and clearly defining a workflow, the average time to start a new study in 2024 was 172 days. A marked decrease from an average of more than 250 days in 2023. This reduction means that critical, and sometimes lifesaving, treatment options are available to Nicklaus patients faster than ever.
To remain competitive with other institutions, it is crucial that NCRI has a diverse research portfolio with a variety of designs and phases. To achieve this, the operations team strengthened relationships with industry sponsors, other institutions, and national groups or consortiums. As a result, the NCRI opened a total of 29 studies 2 authored by Nicklaus, 13 from industry sponsors, 9 authored by other institutions, and 5 from national groups.
From the start, NCRI leadership recognized a significant interest in conducting original research here at Nicklaus. The Research Development Center (RDC) was founded specifically to support internally generated Investigator Initiated Trials (IIT). The RDC is a group of research professionals dedicated to supporting IIT's from inception to close-out including design, funding, contracting, regulatory support, operational support, and much more. Since its inception in February, the RDC has overseen the submission of 77 studies, including but not limited to 6 original observational or interventional studies and 8 compassionate use studies.
Some of the more complex administrative processes supporting research are handled through the Office of Sponsored Programs (OSP), who oversees contracting and financing associated with external funding. In 2024, under the direction of Xonia Cerro, the OSP implemented several initiatives to improve quality and timeliness.
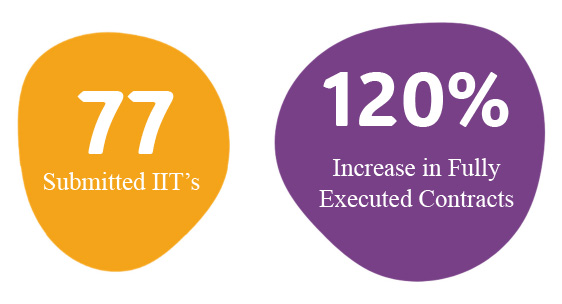
As a result of their efforts, the OSP proudly reports 157 fully executed contracts in 2024, an increase of almost 120% over 2023. This increase is a result of improved contract negotiation timelines in 2024 averaging 52 days. In 2024, the OSP supported 15 research-related grants representing more than $750 thousand in externally funded research. At the opening of a new year, the NCRI is motivated by the success of 2024 and committed to continued growth. Remarking on the anticipated successes, Rebecca Jones said “As we head into 2025, our goal is to help the Nicklaus family understand and engage in therapeutic clinical trials. Clinical trials help us understand diseases; improve detection of acute or chronic illnesses; and ensure discovery or development of medical innovations.”